Think about...
What is the difference between a nucleoside and a nucleotide?
What is the difference between a ribonucleotide and a deoxyribonucleotide?
Structure and Nomenclature of Nucleosides and Nucleotides
Structure: Nitrogenous Bases
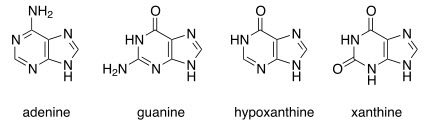
Purines: Adenine, guanine, hypoxanthine, and xanthine
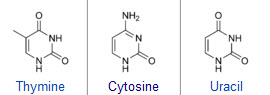
Pyrimidines: Thymine, cytosine, and uracil
Sugars: Ribose and deoxyribose
The prime or (') designation is used when the sugar is attached to the base to distinguish the base atoms from the sugar atoms. Deoxyribose differs from ribose by having a hydrogen in place of a hydroxyl group at carbon 2, as shown below.

Nucleoside: NItrogenous bases + a five-carbon sugar.
A nucleoside is formed by a β-glycosidic bond between the carbon 1' of the sugar and the nitrogen 9 of the purine or nitrogen 1 of the pyrimidine.
Nucleotide:
nitrogenous bases + a five-carbon sugar + one or more phosphates.
A nucleotide is formed when a phosphate group is covalently attached to the carbon 5' of the sugar (Nucleotide = nucleoside + phosphate). Nucleotides can contain one, two, or three phosphate groups.
The diagram below illustrates this. (Although not shown here, the 2' and 3' carbons can also have phosphates attached to them.)



