Nucleotide Metabolism
Prerequisite Material
Purpose: To review and reinforce your knowledge of the basics of nucleotide metabolism.
Recall Objectives:
Please note that we are not assuming that you have memorized the chemical structures of all of the compounds shown here but that you have some idea of what the structures are like and the kinds of things that they do in the normal state.
Later you will learn about how alterations in their metabolism cause a variety of diseases.
What is the difference between a nucleoside and a nucleotide?
What is the difference between a ribonucleotide and a deoxyribonucleotide?
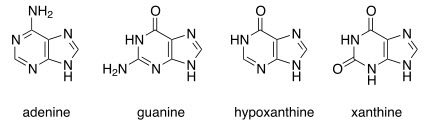
Purines: Adenine, guanine, hypoxanthine, and xanthine

Pyrimidines: Thymine, cytosine, and uracil
Sugars: Ribose and deoxyribose
The prime or (') designation is used when the sugar is attached to the base to distinguish the base atoms from the sugar atoms. Deoxyribose differs from ribose by having a hydrogen in place of a hydroxyl group at carbon 2, as shown below.

Nucleoside: NItrogenous bases + a five-carbon sugar.
A nucleoside is formed by a β-glycosidic bond between the carbon 1' of the sugar and the nitrogen 9 of the purine or nitrogen 1 of the pyrimidine.
Nucleotide:
nitrogenous bases + a five-carbon sugar + one or more phosphates.
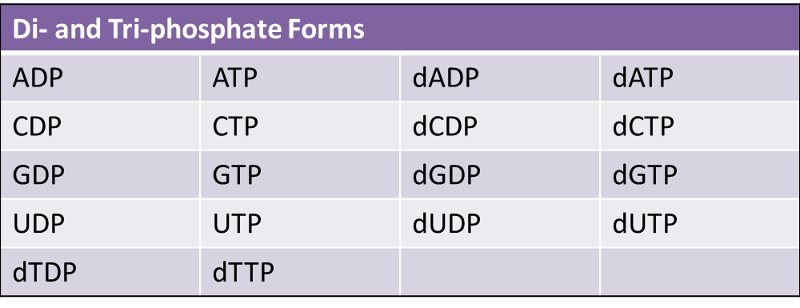
A nucleotide is formed when a phosphate group is covalently attached to the carbon 5' of the sugar (Nucleotide = nucleoside + phosphate). Nucleotides can contain one, two, or three phosphate groups.
The diagram below illustrates this. (Although not shown here, the 2' and 3' carbons can also have phosphates attached to them.)


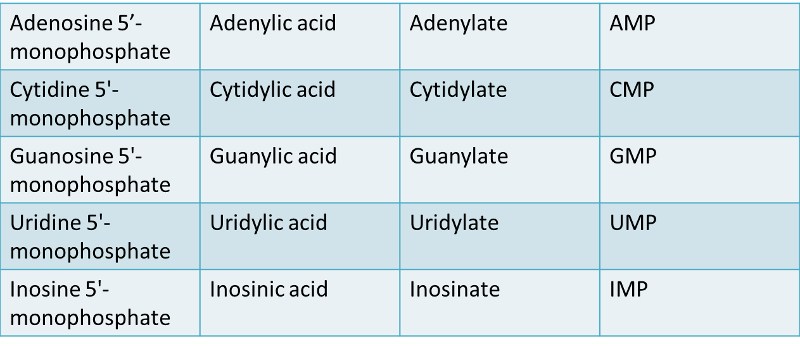
The corresponding deoxyribonucleotides are: dAMP, dCMP, dUMP, dGMP, dTMP.
Note: because ribothymidylic acid is so rare, dTMP is often referred to as TMP.



All images created with resources in the public domain on behalf of the Undergraduate Medical Education office, School of Medicine, University of Texas Health Science Center at San Antonio.