Causes of Mutation
Agents that cause mutations are mutagens. We will focus on mutatgens which can alter the base-pairing properties.
Normal Base-Pairing

Chemical agents
Chemical agents that change normal base-pairing can generate mutations. These chemicals can be further classified according to their mechanism of actions.
A. Chemicals that deaminate amino groups on the bases to keto groups. e.g. A to hypoxanthine (HX), G to xanthine (X), C to U. The resultant products usually will have a different base-pairing property (see information below in the box). HX will base pair with C and U will base pair with A. X has similar base pairing property as G. Deamination can be chemically induced, spontaneous, or Induced by reactive oxygen species that are produced as by-products of oxidative metabolism.

Examples of deamination on the subsequent DNA replication products
 |
Use the flipbook below to view the steps in formation of initiation complex. Turn the pages by clicking on the right or left side of each page. |
|
This content requires JavaScript enabled.
|
|
B. Chemicals that change a base by covalent modifications.The following are some examples.
|
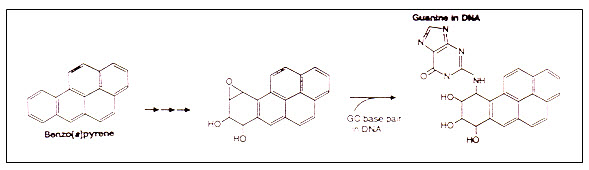
Alkylating agents such as ethylene oxide and Benzo[ a ]pyrene in cigarette smoke or coal tar. Modification of guanine by Benzo[ a ]pyrene would result in a distortion of the DNA helical structure. Upon replication, the DNA product would contain an insertion. |
|
|
|
Reactive oxygen species can modify guanine residues. |
|
Ethylene oxide modifies guanine (G) residues, leading to the formation of ethylguanine (eG) which pairs with thymine. |
An example of the base sequence of the DNA product following a G -> eG mutation:

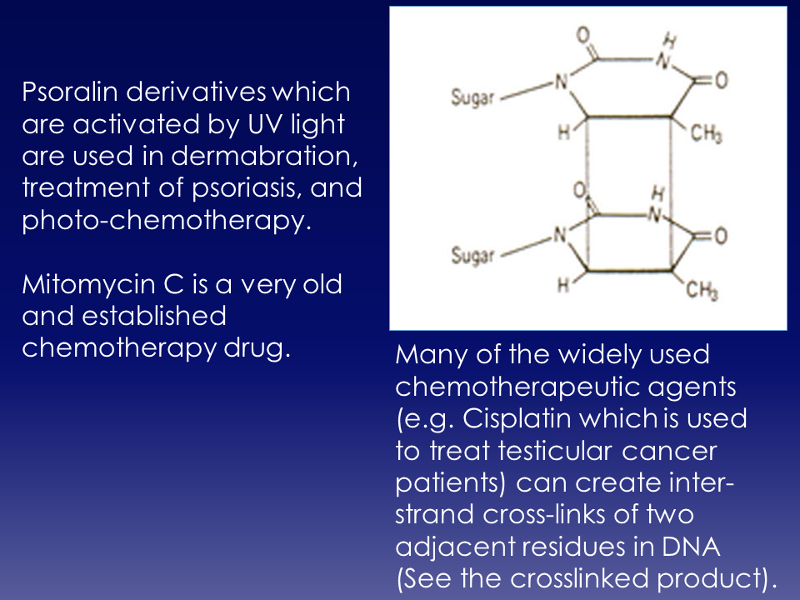
C. Chemicals that cause cross-linking of DNA strands.
Crosslinking of DNA can lead to mutations during subsequent replication or transcription. However, as indicated in the table below, there are several medical treatments that also take advantage of this phenomenon to stop replication of abnormal or cancer cells.
D. Radiation damage
UV and X-ray radiation can result in:
- DNA fragmentation
- Thymidine dimerization - UV light can cause two adjacent thymidine residues in a DNA molecule to form a covalently linked dimer such as the structure shown above, and
- Shift in the equilibrium of the tautomeric forms of bases. The minor tautomers differ from the major forms in their base-pairing properties