
Lipid Metabolism
After completing this activity, you should be able to:
Lipids are a structurally heterogeneous collection of molecules made largely of carbon and hydrogen with a little oxygen, although nitrogen and phosphorus are often thrown in as well. The feature these disparate molecules have in common is that they are hydrophobic.
What is important about hydrophobicity?
A fatty acid is essentially a chain of carbon atoms bound to hydrogen ending in a carboxyl group. They differ from each other in their lengths and in the number and location of carbon-carbon double bonds.
What's the difference between saturated and unsaturated fatty acids?
You can classify fatty acids according to their length:
Very long-chain
Long-chain
Medium-chain
Short-chain
Types of Lipids (continued)
These consist of one, two or three fatty acids connected to glycerol (a carbohydrate) by an ester linkage.


Types of Lipids (continued)
Glycerophospholipids are major constituents of membranes and are responsible for the membrane being a bilayer.
These are more complex structures consisting of a molecule of glycerol to which are attached two fatty acids, a phosphate, and usually one other small molecule (X). The general structure is shown here:
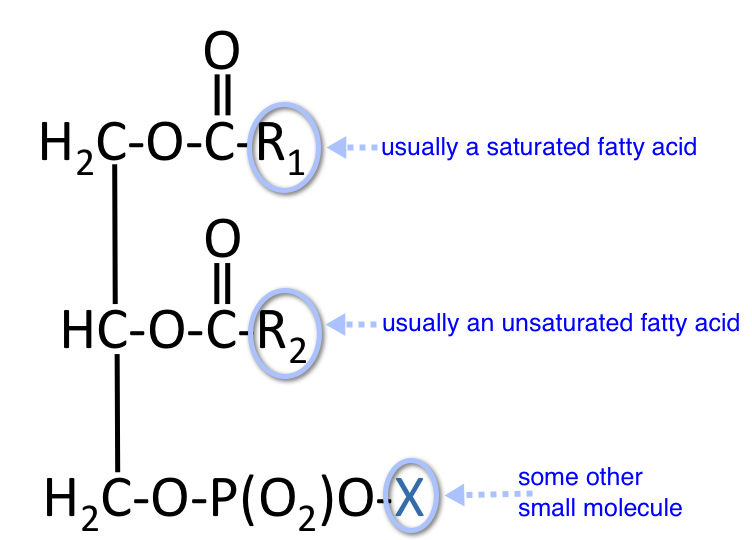
What is diphosphatidylglycerol ?
Types of Lipids (continued)
These are somewhat more complex lipids with a superficial resemblance to glycerophospholipids. All of them contain, instead of glycerol, the fatty acid derivative sphingosine.
Sphingolipids have a fatty acid (sometimes a very long one) attached to the amino group of sphingosine and a compound X attached to the terminal hydroxyl group of sphingosine. Here are some examples:
|
If the X compound is... |
then the sphingolipid is... |
|---|---|
|
H |
ceramide |
|
Phosphocholine |
sphingomyelin |
|
a simple sugar |
a cerebroside or globoside |
|
a complex sugar |
a ganglioside |
Sphingolipids play important roles in certain membranes. They are often involved in signaling. Just like any other biochemical compound, they have to be broken down eventually. Lesions in the degradation pathway of certain sphingolipids can cause very serious diseases such as Tay-Sachs.
Structurally steroids have a "sterane" core, consisting of three 6-carbon rings and a 5-carbon ring. The best-known of the steroids is cholesterol.
Cholesterol is an important constituent of our membranes. It is also the starting point for synthesis of the steroid hormones and bile acids. This topic is not covered within this module.
Most of the fat we eat is in the form of triacylglycerides.
We also consume some cholesterol and glycerophospholipids.
In the adipocytes, the enzyme triacylglycerol lipase releases the fatty acids which are carried by albumin to tissues that need them such as the muscle.
In the muscle the fatty acids are activated by being bound to coenzyme A by the enzyme acyl CoA synthetase that catalyzes the following reaction:
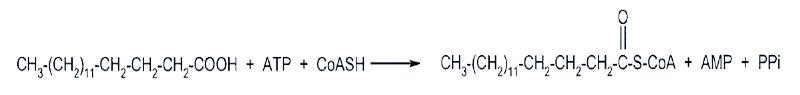
Note that this reaction costs us two ATP equivalents and generates a pyrophosphate. The subsequent hydrolysis of the pyrophosphate renders the reaction irreversible.
At this point, the structure of the fatty acid makes a difference:
![]()
![]()
Digesting Fatty Acids (continued)
The process by which we digest fatty acids is called β-oxidation.

For palmitic acid, for example, the full process can be summarized as follows:
Palmitic acid yields 7 molecules of FADH2, 7 of NADH, and 8 acetyl CoA's. The electrons from FADH2 and NADH are sent into the electron transport pathway of the mitochondria where they yield energy and the acetyl groups are transferred from CoA into the TCA cycle where they too yield energy.
Some fatty acids have an odd number of carbons. Here the last cycle of β -oxidation will give one molecule of acetyl-CoA and one of propionyl-CoA. A pathway requiring Vitamin B12 converts propionyl-CoA into succinyl-CoA, which enters the TCA cycle.
Some fatty acids are unsaturated. Additional enzymes are required to re-arrange the double bonds so that the fatty acid can be digested by β-oxidation. The bottom line is that for every double bond in a fatty acid, one of the cycles will lack the acyl-CoA dehydrogenase step. Hence, one less FADH2 and thus less energy is produced. Another way of putting this is that one gram of butter is more fattening than one gram of olive oil.
Very Long-Chain Fatty Acids are largely oxidized by peroxisomes. These organelles have enzymes analogous to those in mitochondria, except that the acyl dehydrogenase reaction is very different and leads to production of H2O2.
We make fatty acids as well as break them down. Fatty acids are synthesized in the cytosol. The pathway of fatty acid biosynthesis is not just the reverse of fatty acid degradation, although there are some interesting parallels between the two pathways.
Two enzymes synthesize palmitate from acetyl CoA.
In this reaction a carboxyl group is added to acetyl CoA to generate malonyl CoA. This biotin-containing enzyme catalyzes the committed step in fatty acid biosynthesis and is subject to a complex regulation not covered in this activity.
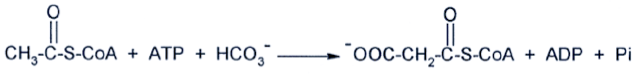
This enzyme is one of the most complex in our bodies. It consists of three polypeptide chains, two of which are identical to each other. The third is a very small polypeptide called acyl carrier protein (ACP), which contains a phosphopantetheine group (derived from the vitamin panthothenic acid) that is identical to the one in Coenzyme A. The other two polypeptides have 7 different enzymatic activities. The enzyme works by first mobilizing malonyl CoA and acetyl CoA (attaching them to ACP). Then the enzyme begins a cycle of reactions in which a fatty acid grows from ACP. At the end of each cycle the growing fatty acid is 2 carbons long. When the fatty acid has reached 16 carbons in length, it is cleaved from the ACP.
The activities of fatty acid synthase are as follows:

The mitochondria and the endoplasmic reticulum are the sites where we make fatty acids longer than 16 carbons.
An enzyme complex in the endoplasmic reticulum inserts double bonds into fatty acids. For example, in the case of stearoyl CoA, a cis-∆9 bond is inserted to form oleoyl CoA:
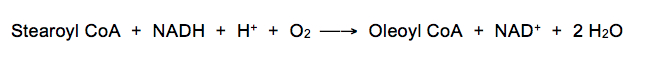

All images are courtesy of Jean Jiang, Ph.D. except as indicated below.
Page 6
"cholesterol": Image cholesterol.svg retrieved from Wikimedia Commons, http://en.wikipedia.org/wiki an original work attributed to BorisTM. The author of this work has released it into the public domain, worldwide.